Life Science Breakthrough: Novartis Kymriah Approved, Launching the CAR T-Cell Therapy Era
In August 2017, Novartis's Kymriah (tisagenlecleucel) received approval from the U.S. Food and Drug Administration (FDA), becoming the first CAR T-cell therapy approved in the U.S. This therapy genetically engineers a patient's own T-cells to recognize and attack cancer cells.
BUSINESSES RESHAPING OUR WORLD
Global N Press
8/27/20171 min read

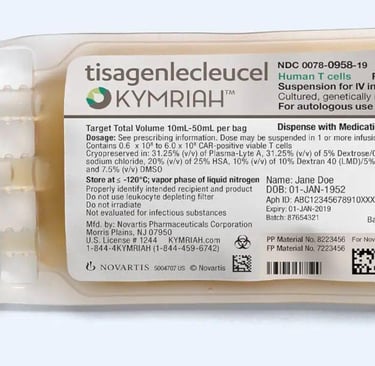
In August 2017, Novartis's Kymriah (tisagenlecleucel) received approval from the U.S. Food and Drug Administration (FDA), becoming the first CAR T-cell therapy approved in the U.S. This therapy genetically engineers a patient's own T-cells to recognize and attack cancer cells.
Kymriah's approval was a milestone in Life Sciences and gene therapy, proving the immense potential of cellular immunotherapy in treating cancer. This event drew unprecedented attention from investment firms to the biotechnology sector, accelerating the commercialization of the future industry of personalized immuno-oncology.